The Octet Rule: Understanding Chemical Bonding in Depth
Written on
Chapter 1: The Octet Rule Explored
The octet rule, often referred to as the rule of eight, acts as a crucial guiding principle in comprehending chemical bonding.
“I am a real Americannnnn” — apologies for the earworm, but it’s a catchy tune! Reflecting on our journey, it’s exciting to acknowledge the first American to make substantial contributions to the field of chemistry. While many Americans have played vital roles, this marks a significant milestone in our exploration. The innovative approach of Lewis in depicting chemical bonds transformed the landscape of chemistry, but that was just the beginning...
For a long time, chemists grappled with the challenge of creating a cohesive theory for bonding in organic compounds. The difficulty stemmed from the fact that organic chemists of the era were primarily recognized as exceptional experimentalists. They had gathered vast amounts of empirical data, which made them less inclined to delve into theoretical frameworks, as they already had a clear understanding of chemical reactivity.

To grasp the significance of this, we must return to the fundamental question: why is chemical bonding important? One of the main attractions of studying chemical bonding is its ability to predict how substances will react with one another. Organic chemists had already mastered the art of predicting reactions, which diminished their motivation to explore theoretical concepts further.
…until Lewis introduced a groundbreaking perspective.
The Emergence of a New Theory
Lewis proposed a shift from the traditional ionic model, where electrons are transferred, to a model where electrons are shared. His innovative approach integrated the concept of eight valence electrons, derived from successful ionic bonding, into his theoretical framework.
In essence, he suggested that organic compounds could be analyzed differently: through the sharing of electrons rather than transferring them.
A brief note on contemporary chemistry: even in compounds deemed highly ionic, there is still an element of electron sharing — no electron is completely transferred. This intriguing nuance has been mathematically validated by some brilliant minds, and we will touch on that shortly.
How does this concept of “electron sharing” function? Let’s consider carbon tetrachloride as an example. The chemical notation for carbon is C, for chlorine it’s Cl, and “tetra” signifies four, leading us to the molecular formula CCl₄. Carbon has four valence electrons, while each chlorine atom possesses seven. In a typical ionic scenario, carbon would need to either lose or gain four electrons, while chlorine would need to gain just one. However, gaining or losing four electrons poses significant challenges for carbon, which is why the shared electrons concept proposed by Lewis comes into play.
Below, you will see the Lewis dot structures for carbon tetrachloride: carbon is depicted in black and chlorine in teal. When they combine to form carbon tetrachloride, carbon shares one electron with each chlorine atom. The pairs of mixed-color electrons represent those shared between carbon and each chlorine atom, while the outer electrons remain unshared.
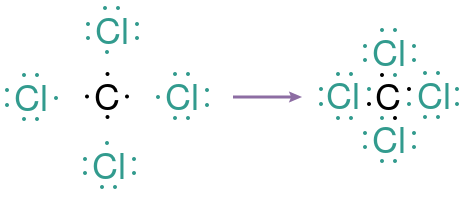
Lewis eventually refined his model to illustrate shared electrons with a line connecting the two atoms involved — this line symbolizes what we now recognize as a “covalent” bond.
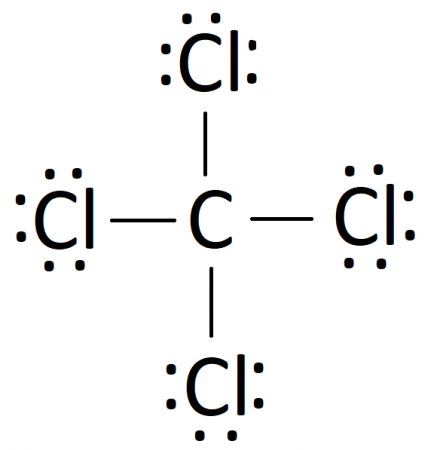
In this illustration, each dot signifies an unshared electron, while each line indicates two shared electrons. A brief calculation shows that every atom in carbon tetrachloride adheres to the octet rule, as shared electrons contribute to this count. This method of representing bonds is highly effective for organic compounds and remains a staple in chemistry classrooms worldwide, despite being conceptualized over a century ago. If this concept sounds unfamiliar, it may be due to my reference to the number eight in chemistry akin to the biblical seven — the “octet rule.” This terminology was popularized by Irving Langmuir in the early 20th century.
The octet rule has become an integral part of chemistry education since I began my studies, and its elegance and unifying nature (applicable to both ionic and covalent compounds) has endured remarkably well. However, there is a significant exception to this rule: hydrogen, an essential element in organic compounds, follows the duet rule instead (which concerns only two electrons). This deviation arises from hydrogen’s small size and low proton count (it has just one!), limiting its capacity to share more than two electrons. Nevertheless, millions of organic compounds primarily adhere to the octet rule.
Moreover, certain other elements may also not conform to the octet rule under specific conditions. It’s essential to recognize that exceptions exist for every rule, including scenarios where hydrogen may not follow the duet rule. For those intrigued by complex concepts, exploring “three-center, two-electron” bonds might be particularly enlightening.
Despite these complexities, Lewis achieved what many thought impossible: he formulated a bonding theory that encompassed both ionic and covalent compounds, establishing a foundational theory for chemistry centered around the significant number eight.
While some dismiss alchemists — the original chemists — as peculiar figures of the past, their legacy lingers on. The emergence of the number eight as a pivotal element in chemical bonding leads me to ponder whether there is something mystical about the field of chemistry after all.

Works Consulted:
Brock, William H. The Chemical Tree: A History of Chemistry. New York: Norton and Co, 2000.
Ihde, Aaron J. The Development of Modern Chemistry. New York: Dover Publications, 1984.
Chapter 2: Revolutionary Insights in Chemistry
The first video titled "The 10 Commandments of Chemistry" provides foundational principles that every chemistry student should understand. It breaks down essential concepts and rules that govern the field, making it a must-watch for anyone interested in chemistry.
The second video, "The 10 Commandments of AP Chemistry," dives deeper into advanced topics crucial for AP students. It outlines significant theories and practices that are vital for mastering AP chemistry.